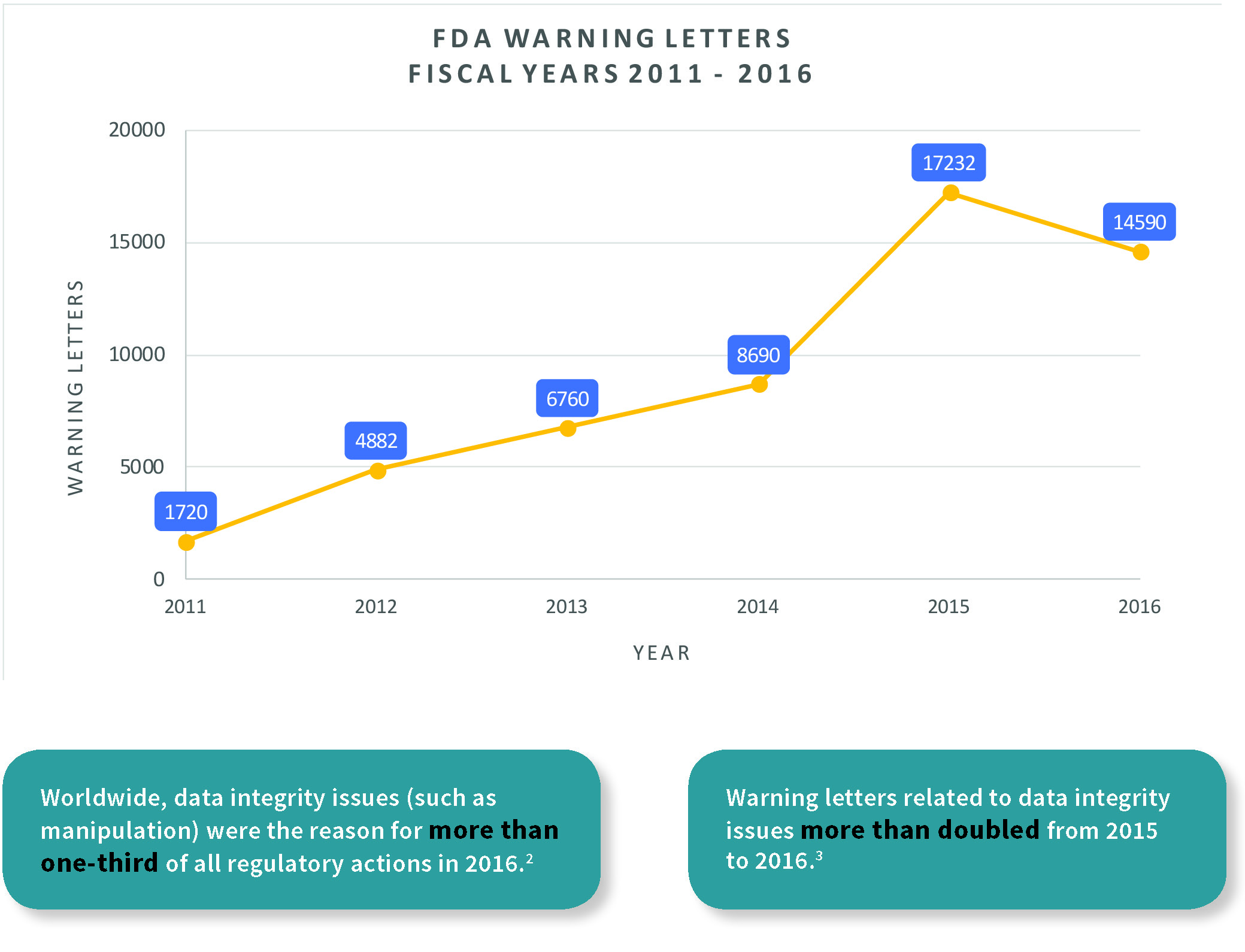
BioInsights - Assuring manufacturing and data integrity for cell and gene therapy regulatory compliance

Data integrity within the biopharmaceutical sector in the era of Industry 4.0 - Alosert - 2022 - Biotechnology Journal - Wiley Online Library
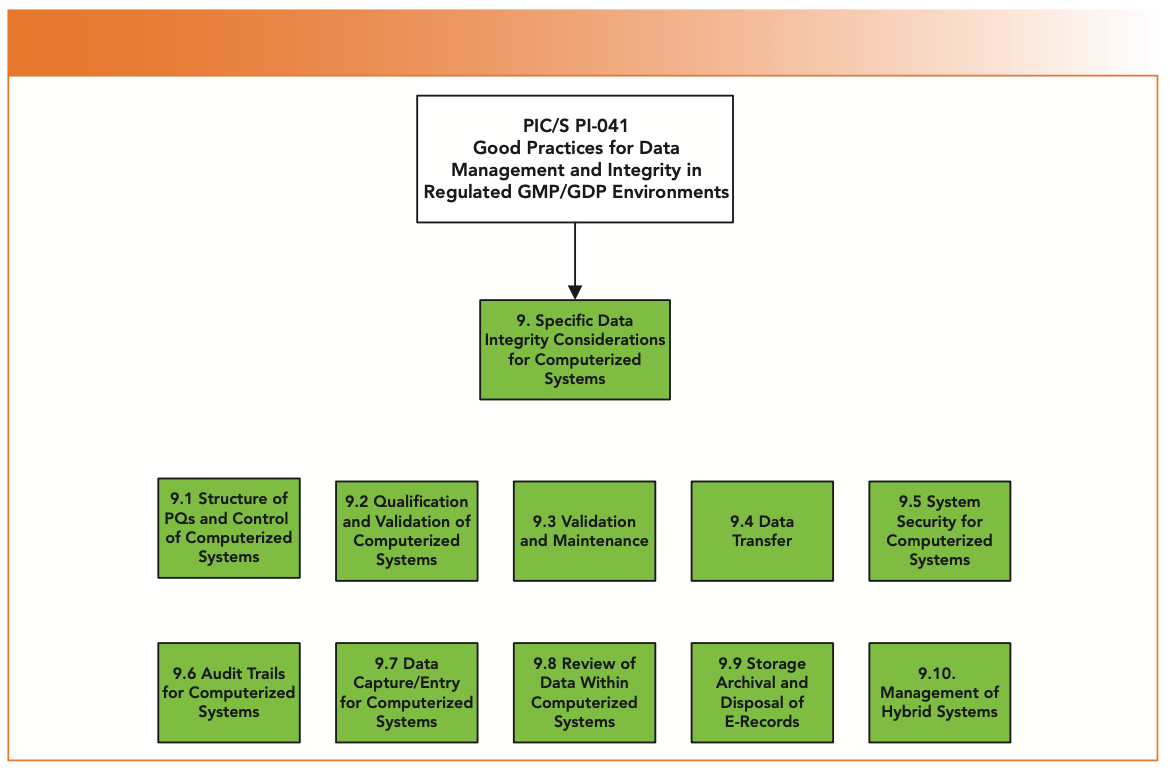
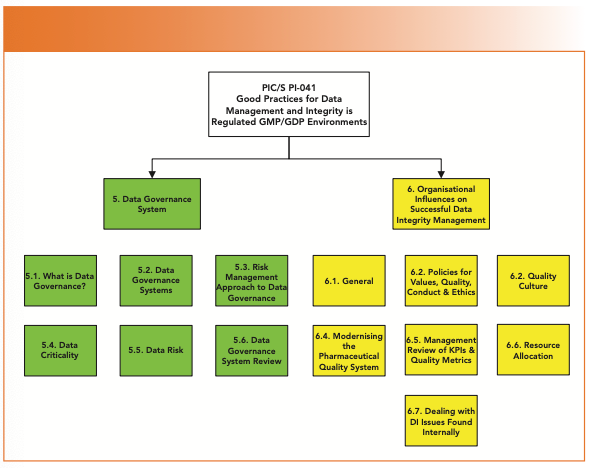
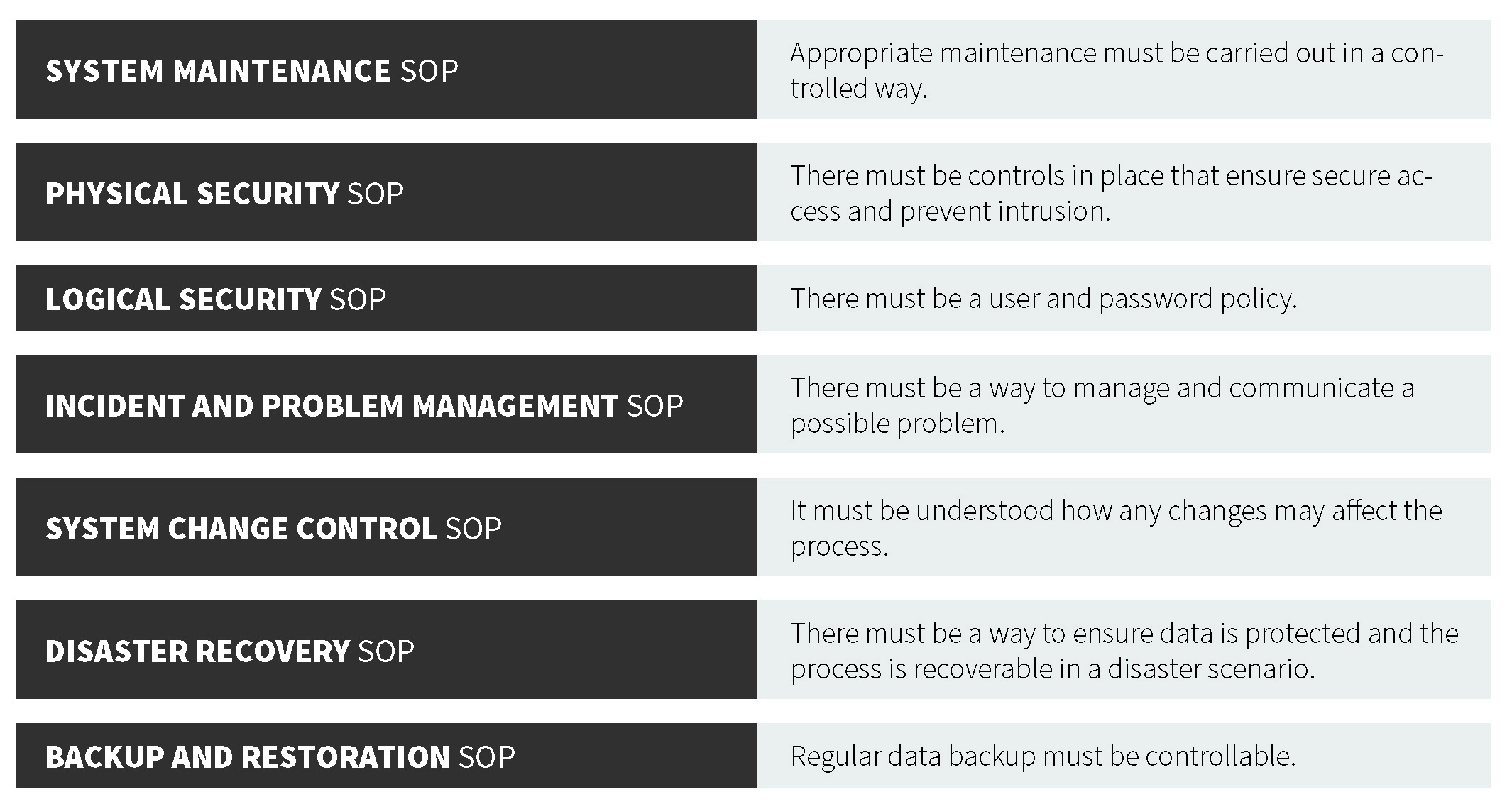
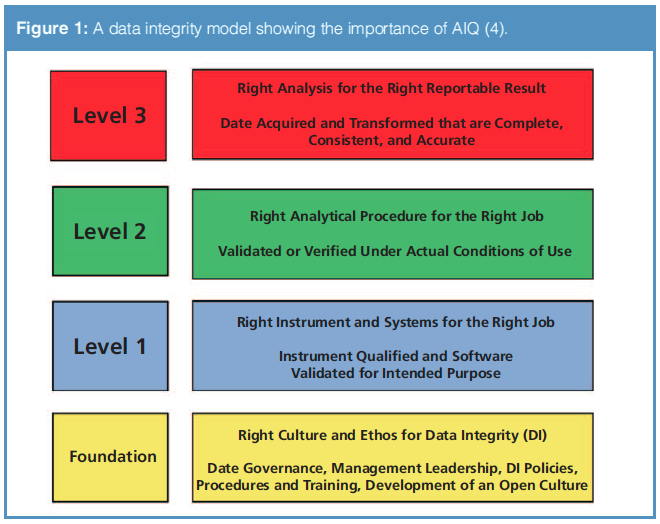
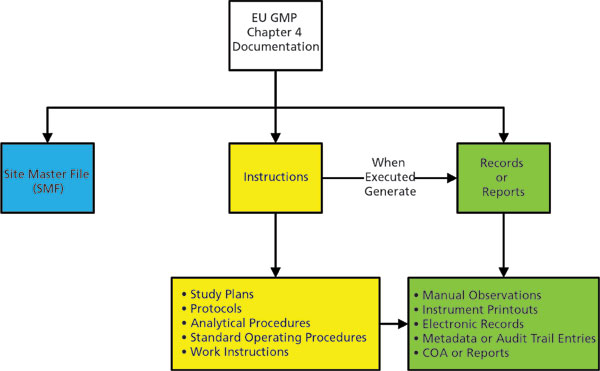
Are You Ready for the Latest Data Integrity Guidance? Part 1: Scope, Data Governance, and Paper Records